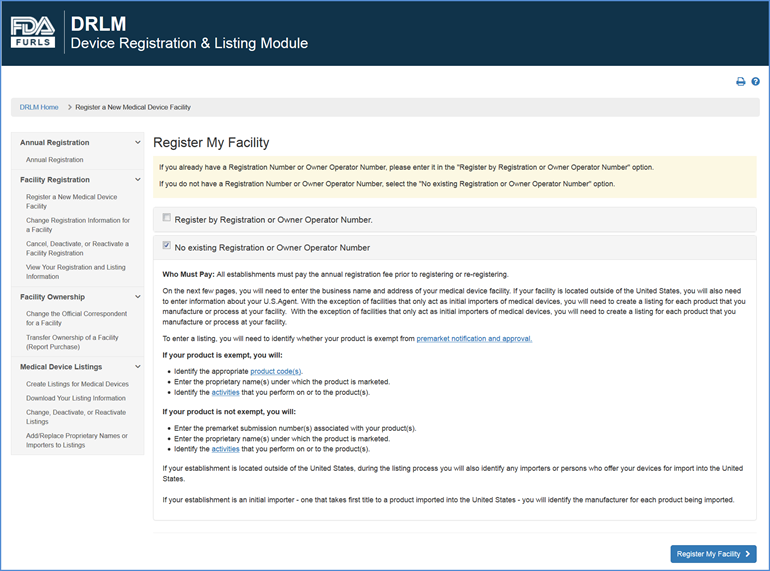
Product code: Fda 2025 importer registration
FDA Registration 2025, Register a New Medical Device Facility Step by Step Instructions 2025, How to Register with FDA FDAHELP.US 2025, FDA Registration FDA Certificate FDA Agent 2025, Register a New Medical Device Facility Step by Step Instructions 2025, U.S. FDA Medical Devices Registration and FDA Device Listing FDA 2025, FDA Registration Product Announcement FDA PMA Product Notice 2025, AI Supplier Monitoring Primority Ltd 2025, FDA Registration Number and other FDA Requirements FDAHELP.US 2025, FDA Registration Product Announcement FDA PMA Product Notice 2025, US FDA Registration is required for your product Understanding 2025, What are the FDA requirements for food USA Food regulations 2025, Foreign Existing Reg in Account 2025, Foreign Existing Reg in Account 2025, Annual Registration 2025, FDA Regulations for Importing Medical Masks to the U.S FDA 2025, Register a New Medical Device Facility Step by Step Instructions 2025, Add Replace Proprietary Names or Importers to Listings 2025, Register a New Medical Device Facility Step by Step Instructions 2025, Diaz Trade Consulting FDA Compliance Food Facility Registration 2025, FURLS Device Registration Listing Initial Registration 2025, FDA Registration Product Announcement FDA PMA Product Notice 2025, FDA Information 2025, How To Register With FDA As An Importer CountyOffice 2025, China import medical device registration certificate CFDA approval 2025, US FDA Medical Device Establishment Registration and Device 2025, The procedure for Import Drug SFDA Registration China CFDA SFDA 2025, Importing Medical Devices 5 Key Factors to Ensure Regulatory 2025, Importing FDA Regulated Products Human Foods YouTube 2025, Add Replace Proprietary Names or Importers to Listings 2025, FDA Proposes New Fees Under FSMA Comment Now Customs 2025, Logistics Plus Receives FDA Importer Registration 2025, US FDA Registration Agents in India and Other Destinations 2025, FDA required registration of foreign food facilities exporting to USA 2025, FDA.2 Royale Business Club International Inc 2025.
FDA Registration 2025, Register a New Medical Device Facility Step by Step Instructions 2025, How to Register with FDA FDAHELP.US 2025, FDA Registration FDA Certificate FDA Agent 2025, Register a New Medical Device Facility Step by Step Instructions 2025, U.S. FDA Medical Devices Registration and FDA Device Listing FDA 2025, FDA Registration Product Announcement FDA PMA Product Notice 2025, AI Supplier Monitoring Primority Ltd 2025, FDA Registration Number and other FDA Requirements FDAHELP.US 2025, FDA Registration Product Announcement FDA PMA Product Notice 2025, US FDA Registration is required for your product Understanding 2025, What are the FDA requirements for food USA Food regulations 2025, Foreign Existing Reg in Account 2025, Foreign Existing Reg in Account 2025, Annual Registration 2025, FDA Regulations for Importing Medical Masks to the U.S FDA 2025, Register a New Medical Device Facility Step by Step Instructions 2025, Add Replace Proprietary Names or Importers to Listings 2025, Register a New Medical Device Facility Step by Step Instructions 2025, Diaz Trade Consulting FDA Compliance Food Facility Registration 2025, FURLS Device Registration Listing Initial Registration 2025, FDA Registration Product Announcement FDA PMA Product Notice 2025, FDA Information 2025, How To Register With FDA As An Importer CountyOffice 2025, China import medical device registration certificate CFDA approval 2025, US FDA Medical Device Establishment Registration and Device 2025, The procedure for Import Drug SFDA Registration China CFDA SFDA 2025, Importing Medical Devices 5 Key Factors to Ensure Regulatory 2025, Importing FDA Regulated Products Human Foods YouTube 2025, Add Replace Proprietary Names or Importers to Listings 2025, FDA Proposes New Fees Under FSMA Comment Now Customs 2025, Logistics Plus Receives FDA Importer Registration 2025, US FDA Registration Agents in India and Other Destinations 2025, FDA required registration of foreign food facilities exporting to USA 2025, FDA.2 Royale Business Club International Inc 2025.